Determination of molar mass using the ideal gas law
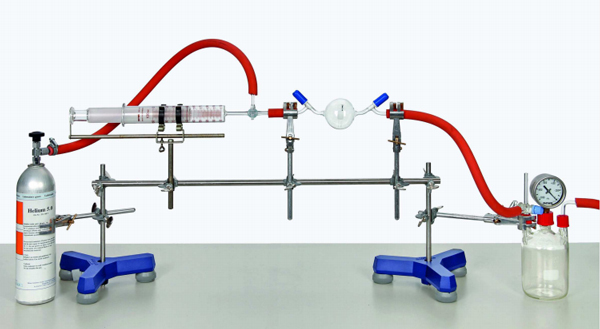
All gases may be considered, to a first approximation, to obey the ideal gas equation which relates the pressure p, volume V, temperature T and amount of substance n of a gas. The amount of gas n is expressed as the number of moles and is equal to m / M where m is the mass of gas present and M is the mass of one mole of the gas. The volume occupied by a known mass of gas is to be measured at a given temperature and pressure, so that the ideal gas equation can be used to estimate the molar mass of the gas.
- Examination of many different gases possible
- Illustrative experimental setup
Rotary valve vacuum pump, two stages, 115 V / 230 V
Glass tubes, right-angled, 10
Glass sphere, 2 stopcocks, 100 ml
Silicon grease Molykote, 50 g
Weather monitor, 6 lines LCD
Rubber tubing,vacuum,i.d.8mm
Glass tube, right-angled
Gas-syringe holder with stop
Compressed gas, nitrogen, 12 l
Right angle boss-head clamp
Fine control valve
Tripod base PHYWE
Hose clip f.12-20 diameter tube
Gas syringe, 100 ml, with 3-way cock
Compressed gas, CO2, 22 g
Universal clamp
Secure bottle, 500 ml, 2 x Gl 18/8, 1 x 25/12
Support rod, stainless steel, l = 250 mm, d = 10 mm
Hose clip, diam. 8-16 mm, 1 pc.
Stopcock,3-way,t-shaped, glass
Adapter for vacuum pump
Compressed gas, methane, 12 l
Rubber tubing,vacuum,i.d.6mm
Spring manometer, 0…-1000 mbar
Support rod, stainless steel, 750 mm
Compressed gas, helium, 12 l
- Molar mass and relative molar mass
- Properties of gases
- Ideal and ordinary gases
- Equations of state
