Titration curves and buffering capacity with Cobra4
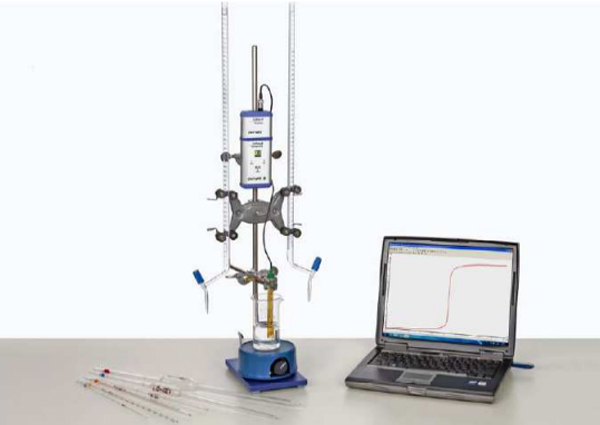
pH values can be measured with the aid of electrochemical measurements and proton-sensitive electrodes (e.g. glass electrodes). By combining a glass electrode with a reference electrode in one housing, a single-rod glass electrode, which is appropriate for acid-base titrations, is created. The titration curves allow an exact determination of the equivalence point in titrations of strong and weak acids and bases.
- Drop counter optimizes the experimental procedure
- Simplified implementation: all pre-settings already prepared
- Extensive automation of the experiment allows concentration on the learning objectives
curricuLAB measureLAB.
Buffer solution, pH 4.62 1000.
Beaker, 100 ml, high-form.
USB charger for Cobra4 Mobile-Link 2 and Wireless/USB-Link.
Retort stand, h = 750 mm.
Volumetric pipette, 25 ml.
Burette, lateral stopcock, Schellbach, 25 ml.
Sodium acetate, anhydr. 25.
Acetic acid, 1 M sol., 1000.
Holder for sensors with support rod.
Rubber caps, 10 pcs.
Pipettor.
Volumetric flask 1000ml, IGJ24/29.
Funnel, glass, top dia. 80 mm.
Buffer solution, pH 9 1000.
Beaker, 250 ml, high-form.
Cobra4 Wireless/USB-Link incl. USB cable.
Right angle boss-head clamp.
Volumetric pipette, 50 ml.
Spoon, special steel.
Caustic soda solution, 1.0 m,
Immersion probe NiCr-Ni, teflon, 300 °C.
Weighing dishes, square shape, 84 x 84 x 24 mm, 25 pcs.
Graduated pipette, 1 ml.
Volumetric pipette, 1 ml.
Magnetic stirrer without heating, 3 ltr., 230 V.
Water, distilled 5 l.
Cobra4 Sensor-Unit Chemistry.
Universal clamp.
Pipette dish.
Volumetric flask 250 ml, IGJ14/23.
Wash bottle, plastic, 500 ml.
Hydrochloric acid, 1.0 mol/l,.
Ortho-phosphoric acid 85% 250.
Beaker, 50 ml, high-form.
Graduated pipette 10 ml.
Volumetric pipette, 2 ml.
Beaker, tall form, 150 ml.
Glycocoll /glycine/ 10.
Magnetic stirring bar 30 mm, cylindrical.
Cobra4 Sensor-Unit Drop Counter.
Burette clamp, roller mount., 2 pl.
Pasteur pipettes, 250 pcs.
Volumetric flask 500 ml, IGJ19/26.
Funnel, glass, top dia. 50 mm.
pH-electrode, plastic body, gel, BNC.
- Strong and weak electrolytes
- Hydrolysis
- Dissociation of water
- Amphoteric electrolytes
- Isoelectric point
- Law of mass action
- Indicators
- Glass electrode
- Activity coefficient
- Buffering capacity
- Henderson-Hasselbalch equation
